 |
 |
| From the Lipid Bilayer to the Fluid Mosaic: A Brief History of Membrane Models |
Phillip Eichman
University of Rio Grande, Rio Grande, Ohio
Membranes are one of the most common features of the biological world. With the exception of some viruses, all living things depend in one way or another on membranes. They surround cells and separate cellular contents from the external environment. Membranes also form special spaces, or compartments, within the cytoplasm that separate various cellular processes. Without membranes, life as we know it would likely not exist.
Today we know a great deal about membranes, but it was not always so. The story of how we came to understand membranes, however, begins not with biology, but with chemistry and the study of lipids (i.e., oils) and how they interact with water.
It has been noted that one of the first to write of the effects of oil on water was Pliny the Elder. In his encyclopedic work, Natural History, Pliny observed that ". . . sea water is made smooth by oil, and so divers sprinkle oil on their face because it calms the rough element. . ." (Tanford 1989). In the centuries that followed the idea that oil calmed troubled waters became a part of folklore.
Likely the first to study this phenomenon scientifically was one not normally associated with the biology of membranes—Benjamin Franklin. Self-trained scientist, author, inven-tor, philosopher, and statesman, Franklin's interests were far ranging. During a stay in England in 1774 Franklin conducted an experiment on the effects of oil on the surface of water. He added a small amount of oil to the water in a small pond in Clapham Common. Immediately he noticed that the oil spread in a thin film over the surface of the water until a large portion of the pond was "smooth as a looking glass" (Tanford 1989). Although later published in Philosophical Transactions of the Royal Society, Franklin's experiment passed largely unnoticed. A little more than a century later, Franklin's experiment was repeated by Lord Raleigh. Raliegh, originally John William Strutt, had attended Cambridge University, majoring in mathematics and physics. After graduation he held various scientific appoint-ments, including the prestigious Professor of Natural Philosophy at the Royal Institution in London.
In 1890 Lord Raleigh conducted a series of quantitative experiments with oil and water. He was able to carefully measure the area to which a known volume of oil would expand and also calculated the thickness of the oil film (Tanford 1989).
His results were published, but noticed by only a few experts in the field. The following year, however, he received a letter from a German woman named Agnes Pockels, describing some experiments that she had conducted in her kitchen. Agnes Pockels, it seems, had developed on her own with little training and support from others in the scientific establishment, a device for carefully measuring the exact area of an oil film. Lord Raleigh assisted Agnes Pockels in publishing her results, the first of fourteen scientific articles she published. Her greatest contribution to science, however, was likely the device that she invented, which is still used today by chemists and physicists studying surface phenomena (Tanford 1989).
At about the same time that Lord Raleigh was experimenting with oil films, Charles Ernest Overton was working on a doctoral degree in botany at the University of Zurich. As it happened, Overton discovered quite acciden-tally, some important properties of membranes. His research was related to heredity in plants and in order to complete his studies he needed to find substances that would be readily absorbed into plant cells. He found that the ability of a substance to pass through the membrane was related to its chemical nature. Nonpolar substances, Overton discovered, would pass quickly through the membrane into the cell. This discovery was quite contrary to the prevalent view at the time that the membrane was impermeable to almost anything but water.
Based on his studies of how various molecules pass through the membrane, Overton published a preliminary hypothesis in which he proposed: (1) that there are some similarities between cell membranes and lipids such as olive oil, and (2) that certain molecules (i.e., lipids) pass through the membrane by "dissolving" in the lipid interior of the membrane. Today, we realize the significance of Overton's hypothesis. At the time, however, there was considerable opposition to Overton, and his ideas (Tanford 1989).
Further research on the nature of oil films was conducted by Irving Langmuir. Trained in physical chemistry, Langmuir worked in the laboratories of General Electric doing research on molecular monolayers. His research eventually turned to lipids and the interaction of oil films with water. Using an improved version of the apparatus originally developed by Agnes Pockels (generally referred to today as a Langmuir trough), he was able to make careful measurements of surface areas occupied by known quantities of oil.
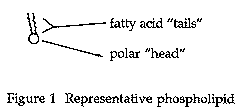
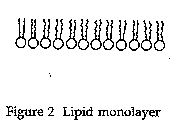
Langmuir published only one paper on molecular monolayers (Langmuir 1917). He proposed that the fatty acid molecules form a monolayer by orienting themselves vertically with the hydrocarbon chains away from the water and the carboxyl groups in contact with the surface of the water. As it turns out, this was a key piece in the puzzle of understanding lipid bilayers and membranes as well. In Figures 1 and 2, this is illustrated with phospholipids, a component of cell membranes.
 |
 |
In their classic experiment, Gorter and Grendel extracted the lipids from red blood cells with acetone and other solvents. Using a modified trough, similar to Langmuir, they were able to demonstrate that lipid molecules could form a double layer, or bilayer (Figure 3), as well as a monolayer.
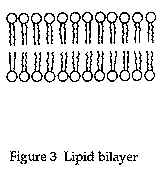 Further, they were able to show that the surface area of the lipids extracted from the red blood cells was about twice the surface area of the cells themselves.
Further, they were able to show that the surface area of the lipids extracted from the red blood cells was about twice the surface area of the cells themselves.
Based on these two observations (i.e., that lipid molecules can form bilayers, and that the surface area of the monolayer extracted from the cells is approximately equal to twice the surface area of the cells) and repeated studies with red blood cells from several animals (human, rabbit, dog, guinea pig, sheep, and goat) Gorter and Grendel concluded that "chromocytes [red blood cells] are covered by a layer of fatty substances that is two molecules thick" (Gorter and Grendel 1925). Although not generally thought of as a "model" of cell membranes, Gorter and Grendel did describe a plausible structure for the membrane.
It is perhaps interesting to note in passing that later researchers discovered that the work of Gorter and Grendel was hampered by the poor techniques of the time. They actually did not completely extract the lipids and also underestimated the surface area of the red blood cells. As it turned out, the two errors essentially canceled each other out and their conclusions were basically correct (Sadava 1993).
The first membrane model to be accepted by the majority of scientists was proposed by Danielli and Davson in 1935. James F. Danielli was trained as a physical chemist, although much of his work was related to biology. His doctoral research was in the area of surface properties of lipids and following graduation he worked at Princeton University from 1933-35 with E. Newton Harvey, an expert in the area of cell surface studies. Working in Harvey's lab, Danielli had found that proteins could be adsorbed to oil droplets obtained from mackerel eggs (Stein 1986). This finding would later become a major component of the membrane model proposed by Danielli and Davson.
In 1935 Danielli returned to University College in London where Hugh Davson, a physiologist, was working. It was through the association of these two that the first membrane model originated.
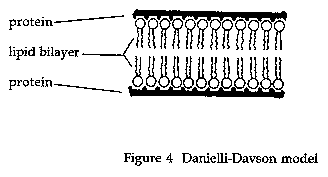 The model proposed by Danielli and Davson (Figure 4) was basically a "sandwich" of lipids (arranged in a bilayer although no mention of Gorter and Grendel was made in the initial paper) covered on both sides with proteins (Danielli and Davson 1935). Later versions of the model included "active patches" and protein lined pores (Danielli 1975).
The model proposed by Danielli and Davson (Figure 4) was basically a "sandwich" of lipids (arranged in a bilayer although no mention of Gorter and Grendel was made in the initial paper) covered on both sides with proteins (Danielli and Davson 1935). Later versions of the model included "active patches" and protein lined pores (Danielli 1975).
This was the basic model for membrane structure accepted by biologists for many years. It was, however, inadequate in explaining many of the findings of later research (Hendler 1971). In 1957 J. D. Robertson proposed a modified version of the membrane model, based primarily on electron microscope studies, which he called the "unit membrane" (Robertson 1957).
Under the high magnification of the transmission electron microscope, membranes have a characteristic "trilaminar" appearance consisting of two darker outer lines and a lighter inner region. According to the unit membrane model, the two outer, darker lines are the protein layers and the inner region the lipid bilayer.
During the 1960s and early 1970s, textbooks generally included an electron micrograph illustrating the "unit membrane," and biologists described the membrane as a "lipo-protein sandwich." It was not to last however, because a major change in the understanding of membrane structure was underway (Hendler 1971).
The unit membrane model was eventually replaced in the early 1970s by the current model of the membrane. This model, known as the fluid mosaic model, was proposed by biochemists S. J. Singer and Garth L. Nicolson (Singer and Nicolson 1972). The model (Fig. 5)
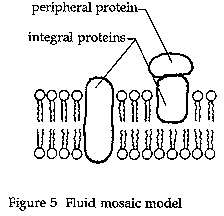 retains the basic lipid bilayer structure first proposed by Gorter and Grendel and modified by Danielli and Davson and Robertson. The proteins, however, are thought to be globular and to float within the lipid bilayer rather than form the layers of the sandwich-type model.
retains the basic lipid bilayer structure first proposed by Gorter and Grendel and modified by Danielli and Davson and Robertson. The proteins, however, are thought to be globular and to float within the lipid bilayer rather than form the layers of the sandwich-type model.
The hydrophobic tails of the phospholipids, the major lipid component of the membrane, face inward, away from the water. The hydrophilic heads of the phospholipids are on the outside where they interact with water molecules in the fluid environment of the cell. Floating within this bilayer are the proteins, some of which span the entire bilayer and may contain channels, or pores, to allow passage of molecules through the membrane. The entire membrane is fluid—the lipid molecules move within the layers of the bilayer while the "floating" proteins also freely move within the bilayer.
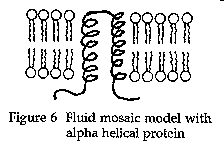
The nature of these membrane proteins was studied by Unwin and Henderson (1984). They found that the portion of the protein that spans the lipid bilayer is hydrophobic in nature (i.e., similar to the lipids forming the bilayer) and arranged in a three-dimensional shape, often in the form of an alpha helix (Figure 6).
 Since 1972, the fluid mosaic model has been modified because of more recent discoveries, such as those of Unwin and Henderson, but it still remains the model preferred by biologists today. It explains the current knowledge of membrane structure and also serves as the basis for our understanding of how membranes function. It has a long history—back at least to the time of Pliny. Like most of modern science, the fluid mosaic model resulted from the cumulative work of numerous individuals. Current biology textbooks, however, generally ignore the historical development and present the model almost as if it were a recent discovery. There are a few textbooks that do contain historical details and teachers wanting more information can consult these (Avilia 1995; Becker, et al. 1996; Mader 1996). Although it may not be covered in your particular textbook, with a little extra effort and imagination, however, you can enliven your class and bring your students a little closer to these pioneer scientists of the past by including some of this history.
Since 1972, the fluid mosaic model has been modified because of more recent discoveries, such as those of Unwin and Henderson, but it still remains the model preferred by biologists today. It explains the current knowledge of membrane structure and also serves as the basis for our understanding of how membranes function. It has a long history—back at least to the time of Pliny. Like most of modern science, the fluid mosaic model resulted from the cumulative work of numerous individuals. Current biology textbooks, however, generally ignore the historical development and present the model almost as if it were a recent discovery. There are a few textbooks that do contain historical details and teachers wanting more information can consult these (Avilia 1995; Becker, et al. 1996; Mader 1996). Although it may not be covered in your particular textbook, with a little extra effort and imagination, however, you can enliven your class and bring your students a little closer to these pioneer scientists of the past by including some of this history.
References